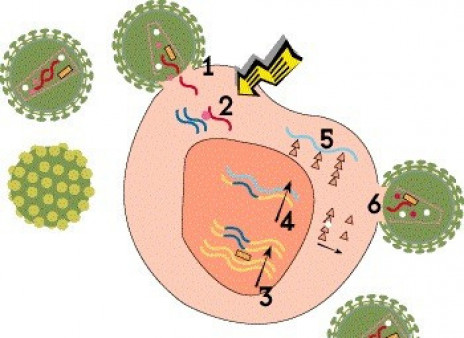
Viruses are not technically alive. In order to reproduce, or replicate, they must hijack cells in the body and use them as “factories” to produce new virus. Compared with other viruses, HIV has an unusual and complex life cycle with many steps that can be blocked by antiretroviral drugs.
Step 1: Cell Entry
For HIV to enter a cell, proteins on the outer envelope of the virus must attach to receptors on the cell surface. HIV primarily binds to CD4 receptors on certain T cells (dubbed CD4 or helper cells). In addition, it must bind to an additional coreceptor. Most strains of HIV use the CCR5 coreceptor, but some can use another coreceptor called CXCR4. After attachment, HIV fuses with the cell and enters it.
Entry inhibitor drugs interfere with this process. The attachment inhibitor fostemsavir (Rukobia) binds to HIV’s gp120 envelop protein and prevents it from attaching to CD4 receptors, while ibalizumab (Trogarzo) binds to CD4 itself. Maraviroc (Selzentry) blocks CCR5 receptors. Some experimental gene therapy approaches aim to delete CCR5 receptors from cells, thereby blocking viral entry. Enfuvirtide (Fuzeon) prevents HIV from fusing with host cells.
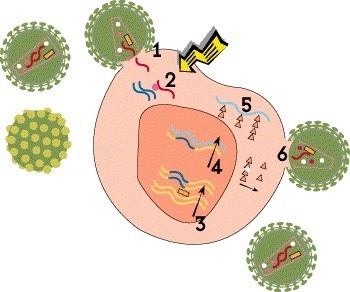
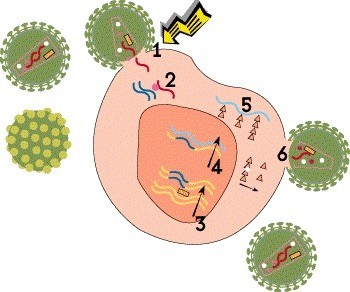
Step 2: Reverse Transcription
Once HIV is inside the cell, its cone-shaped inner shell, known as the capsid, breaks down, releasing the viral genetic material and essential enzymes. Unlike most viruses, HIV stores its genetic blueprint in the form of RNA, which it must convert to DNA in order to multiply. This process, known as reverse transcription, is carried out by a polymerase enzyme called reverse transcriptase. Using RNA as a template, the enzyme creates a strand of DNA by sequentially adding four nucleotide building blocks: adenine, cytosine, guanine and thymine.
Many antiretroviral drugs are reverse transcriptase inhibitors that block the conversion of RNA into DNA. Nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs), also known as nucleoside or nucleotide analogues, are defective building blocks. When these are added to the growing DNA chain instead of a natural nucleotide, the copying process comes to a halt. The oldest HIV drugs, such as AZT (Retrovir) and ddI (Videx), are nucleoside analogues. Tenofovir (Viread [TDF] or Vemlidy [TAF]) is a nucleotide analogue, which requires less processing in the body. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), such as rilpivirine (Edurant and Cabenuva) and doravirine (Pifeltro), bind to the enzyme itself and block its activity.
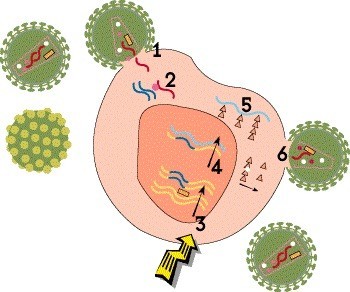
Step 3: Integration
The newly created HIV DNA then moves into the cell’s nucleus, where a viral enzyme known as integrase inserts it into human DNA in chromosomes. Then, when the cell tries to use its DNA to make its own proteins, it produces viral proteins as well. This integrated HIV blueprint, known as a proviral DNA or a provirus, can lie dormant in resting CD4 cells indefinitely during treatment, but it can start churning out new virus if the drugs are stopped. This latent viral reservoir is a major barrier to a cure.
HIV meds called integrase inhibitors block the integration step. This drug class includes, for example, bictegravir (in Biktarvy), cabotegravir (Cabenuva and Apretude) and dolutegravir (Tivicay).
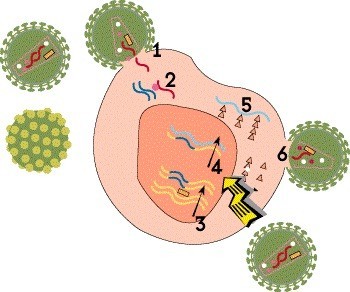
Step 4: Transcription
Once the HIV DNA is integrated into the host cell’s chromosomes, it can be used as a blueprint to make viral proteins. First, the proviral DNA is used as a template to create strands of messenger RNA (mRNA)—a process known as transcription—which serve as instructions for producing the proteins that make up new virus particles.
There are no approved medications that block this step, but HIV cure researchers are exploring various strategies related to viral integration, latency and transcription in the hope of bringing about long-term remission.
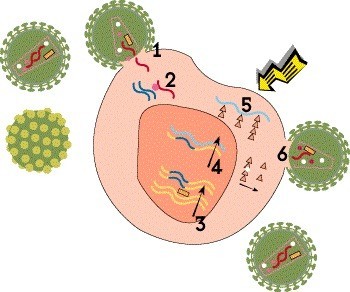
Step 5: Translation
The next step is translation, in which the newly created mRNA is in turn used as a template to produce viral proteins. This process gives rise to all the structural proteins, envelope proteins and enzymes needed to assemble new virus particles, known as virions.
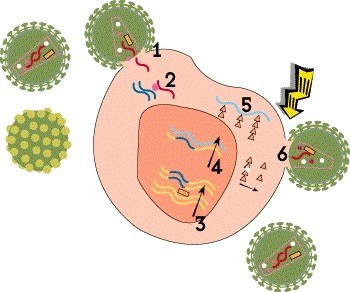
Step 6: Assembly, Budding and Maturation
The final step involves assembly, maturation and release of the new virus. First, the long proteins translated from mRNA must be cut up into smaller pieces that can be used to construct a virus particle. This is done by HIV’s protease enzyme. These component parts are then assembled into a complete but noninfectious virion that buds out of the host cell, taking part of the cell’s outer membrane with it. Finally, the new virus undergoes maturation, or biochemical changes that enable it to infect other cells.
Protease inhibitors, such as darunavir (Prezista), prevent the protease enzyme from cutting up newly created viral proteins into usable pieces. Lenacapavir (Sunlenca), the only HIV capsid inhibitor, interferes with viral assembly, formation of the capsid and release of new virions from the cell. HIV assembly and maturation inhibitors are under study, but none are currently approved.
Combination Therapy
When antiretroviral medications are used alone—or when meds that work the same way are used together—HIV can develop drug resistance that makes them less effective. The virus can mutate to evade almost any single class of antiretrovirals, but this is much more difficult when drugs that work in different ways are used together.
In the mid-1990s, the advent of protease inhibitors and NNRTIs made it possible to put together combination regimens that were finally able to keep HIV in check. Since then, treatment has become simpler and more convenient, but it still requires combining drugs that target two or more steps of the viral life cycle.
Last Reviewed: March 3, 2023